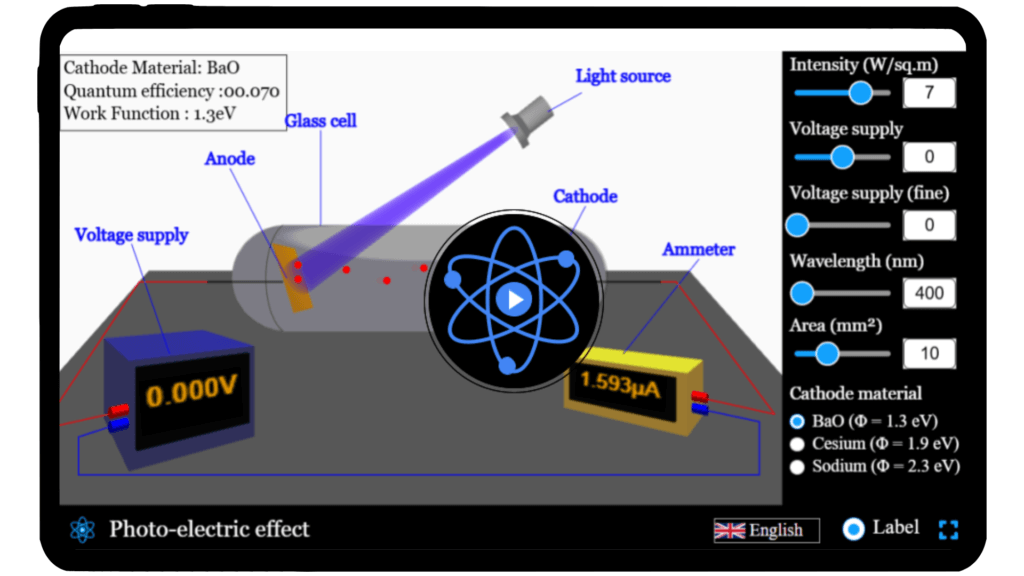
Photo-electric effect simulator
Explore the photoelectric effect and experiment with different light intensities and frequencies to observe how electrons are emitted from a metal surface using our interactive simulator.
Photo-electric effect
Light is more than just what we see — it’s energy, and it can even knock electrons right out of a metal surface! That’s the photoelectric effect, one of the most groundbreaking discoveries in modern physics that helped Einstein win the Nobel Prize.
With our Photoelectric Effect Simulator, you can explore how light energy interacts with matter at the quantum level. Adjust the frequency and intensity of light, experiment with different metals, and observe how electrons are emitted in real time.
Discover how Einstein’s photoelectric experiment revealed the particle nature of light and laid the foundation for technologies like solar cells, LEDs, and digital cameras. Step into the quantum world — experiment, visualize, and learn how light turns into electricity!
\( h\nu = \phi + K_{\text{max}} \)
Mathematical description
Formula describes the relationship between the energy of incident light photons and the kinetic energy of the electrons they emit from a metal surface.
where:
- \( h \) is the Planck’s constant
- \( v \) is the frequency of light
- \( \phi \) is the work function of the metal (minimum energy required to eject the electron)
- \( K_{\text{max}} \) is the maximum Kinetic energy of the electron
Simulator
Dive into the quantum nature of light (photons) with our interactive photoelectric effect simulator!
Interactive Physics Simulator – Photoelectric Effect
🌟 You May Also Like
Suggested experiments and activities based on your progress...
FAQs on Photoelectric Effect
Qus 1. What is the Photoelectric effect?
The photoelectric effect is the emission of electrons from a material when light shines on it. This phenomenon occurs when photons hit the surface of a metal, transferring their energy to the electrons and causing them to eject from the surface of the metal. It played a key role in developing quantum mechanics and earned Albert Einstein the Nobel Prize in Physics.
Qus 2. How does the Photoelectric effect work?
The photoelectric effect works when photons with sufficient energy strike the surface of a metal, transferring their energy to electrons. If the photon energy is higher than the metal’s work function, electrons are ejected. The energy of the emitted electrons depends on the frequency of the light.
Qus 3. What factors affect the Photoelectric effect?
Several factors affect the photoelectric effect, including the frequency and intensity of the incident light, and the type of material (specifically its work function). The most critical factor is the frequency of light, which must exceed the threshold frequency for electron emission to occur.
Qus 4. What is the equation for Photoelectric effect?
Einstein’s photoelectric effect:
\( E = h\nu = \phi + K_{\text{max}} \)
- \( E \) is the energy of the photon
- \( h \) is the Planck’s constant
- \( v \) is the frequency of light
- \( \phi \) is the work function of the metal (minimum energy required to eject the electron)
- \( K_{\text{max}} \) is the maximum Kinetic energy of the electron
Qus 5. What is the threshold frequency in the Photoelectric Effect?
The threshold frequency (\( \nu_o \)) is the minimum frequency of light required to eject electrons from a metal in the photoelectric effect.
\( E = h\nu_o \)
When the energy associated with the threshold frequency is absorbed by the electrons in the metal, it is just sufficient to overcome the attractive forces holding them within the metal, allowing them to escape its surface. If the light’s frequency falls below this threshold, no electrons will be emitted, regardless of the light’s intensity.
The energy associated with the threshold frequency is also referred to as work function. Every metal has a different work function. This concept highlights the particle nature of light.
Qus 6. What is the effect of voltage in photoelectric experiment?
In the photoelectric experiment, the voltage applied between the metal surface (cathode) and the collector (anode) affects how photoelectrons behave after being emitted.
- Stopping Voltage (Negative/Retarding Voltage)
If you apply a negative voltage to the collector (anode), it repels the emitted photoelectrons.
As the negative voltage increases, fewer electrons reach the anode.
At a certain voltage, called the stopping potential (V₀), even the most energetic electrons can’t reach the anode, and current drops to zero.
This stopping potential helps calculate the maximum kinetic energy of the emitted electrons:
\begin{equation}
K_{\text{max}} = e.V_o
\end{equation}
- Zero Voltage
If there’s no external voltage, photoelectrons still move from the cathode to the anode if they have enough kinetic energy.
You’ll get a small current due to naturally energetic electrons
- Positive Voltage (Accelerating Voltage)
A positive voltage attracts the electrons, accelerating them toward the anode.
Even low-energy electrons can now contribute to the current.
The photoelectric current increases until all emitted electrons are collected (saturation current).
Qus 7. What do you mean by "current saturation" Photoelectric effect?
In the photoelectric effect, increasing the positive voltage between the cathode and anode helps more of the emitted electrons—especially the slower ones—reach the anode, which increases the current. However, after a certain point, increasing the voltage doesn’t cause more electrons to be emitted from the metal. This is when the current reaches a saturation point. To increase the current any further, the intensity of the incoming light needs to be increased.
Qus 8. What are the applications of the Photoelectric effect?
Applications of the photoelectric effect include solar cells, where sunlight is converted into electricity, and photodetectors used in cameras and automatic doors. It’s also used in the study of materials and quantum physics, influencing advancements in modern technology like light sensors.
Qus 9. Why is the Photoelectric effect important?
The photoelectric effect is crucial for understanding quantum mechanics. It demonstrated that light can behave as both a particle and a wave, supporting the idea that energy is quantized. This discovery also led to advancements in technologies like solar panels, photodetectors, and electron microscopes.
Qus 10. How did the Photoelectric Effect challenge classical physics?
Classical physics predicted that the intensity of light should affect electron emission, but the photoelectric effect showed that frequency, not intensity, determines whether electrons are ejected. This observation proved the particle nature of light by confirming that light is made up of packets of energy (called photons) and the energy of each photon depends only on the frequency of the light. This experiment led to the development of theory of dual nature of light, which was further used to for development of quantum mechanics.
Qus 11. What is the difference between the Photoelectric Effect and the Compton Effect?
The photoelectric effect involves the ejection of electrons from a material when exposed to light, while the Compton effect involves the scattering of X-rays or gamma rays, resulting in a change in wavelength. Both effects demonstrate the particle nature of light, but they occur under different conditions.
Qus 12. How is the Photoelectric Effect used in solar panels?
In solar panels, the photoelectric effect is harnessed to convert sunlight into electrical energy. Photons from sunlight strike semiconductor materials like silicon, knocking electrons free and creating a flow of electric current. This process is fundamental to the operation of photovoltaic cells in solar energy systems.

Why, despite the photoelectric effect being discovered long ago, haven’t solar panels become a common standard yet?
Hi Bharti,
Although the photoelectric effect was found over a century ago, solar panels have not yet become standard practice because it is difficult to effectively convert sunlight into usable electricity. Solar panels utilize the photovoltaic effect, in which light produces electron-hole pairs in a semiconductor, in contrast to simply ejecting electrons in the photoelectric effect. Efficiency is limited—typically only 15–25% of sunlight is converted to electricity—by reflection, heat dissipation, and carrier recombination. Additional factors of high installation costs, use of space, and intermittency of sunlight that necessitates storage mechanisms hamper large-scale adoption. While solar power is becoming increasingly recognized, realistic, reliable, and cost-effective deployment still faces these physical and financial constraints.
Hi,
Do you also have solar cell experiment?
Hi Anuj,
We’re currently developing a solar cell simulator and will let you know once it’s ready.
Hi, You can explore the solar cell experiment here: https://explerify.com/simulator-solar-cell